On December 09, 2009, Osteologix Inc. (OLGX.OB) announced that it should be prepared to file a marketing application in 2011 in the European Union for its proprietary strontium therapy, NB S101 (strontium malonate). If successful, this will be Osteologix' first marketing approval of NB S101 for the treatment of postmenopausal osteoporosis. Following the successful completion of the company's initial Phase 2 study comparing NB S101 (strontium malonate) to Protelos (strontium ranelate), which is marketed by Les Laboratoires Servier (Servier), Osteologix sought feedback on its development program from various regulatory authorities. Osteologix has determined that the company will be positioned to pursue a European Marketing Application for NB S101 as a treatment for post-menopausal osteoporosis. The company expects that it can complete the additional development work in 2010 and will be prepared to file a marketing application during the first half of 2011. This pathway is in keeping with the Company's overall strategy of developing its once-daily strontium tablet for the osteoporosis market. Osteologix has initiated discussion with potential regional and global partners and believes that the selected partner(s) will be able to begin marketing the product by 2012, providing them with at least 12 years of patent protection in the European Union.
This is an abbreviated version of a press release by Osteologix. See the original at www.osteologix.com.
Skeleton Pirate

Artist: LindaB
WELCOME TO STRONTIUM FOR BONES BLOG
Have you experienced negative, and even dangerous, side effects from Fosamax (alendronate), Boniva (ibandronate), Actonel (risedronate), Reclast (zoledronic acid), Prolia (denosumab), Forteo (teriparatide), Tymlos (abaloparatide), or other drugs prescribed for osteoporosis? If you have, then rest assured there is a safe, effective treatment for this condition. Strontium, primarily in the form of strontium citrate, is taken orally once a day.
Visitors to my blog can leave comments or ask questions and can remain anonymous, if they wish. Their comments are relayed to my g-mail inbox. Below each post, the number of comments for that post is cited and underlined because it is a link. By clicking on that link below any post, a window opens so that a visitor can leave a comment. Ideally, visitors leave comments on posts most relevant to their comments. All comments to my posts are moderated by me.
Browse the posts and visit the link library of references.
Visitors to my blog can leave comments or ask questions and can remain anonymous, if they wish. Their comments are relayed to my g-mail inbox. Below each post, the number of comments for that post is cited and underlined because it is a link. By clicking on that link below any post, a window opens so that a visitor can leave a comment. Ideally, visitors leave comments on posts most relevant to their comments. All comments to my posts are moderated by me.
Browse the posts and visit the link library of references.
Blog Archive
Tuesday, December 29, 2009
Friday, November 6, 2009
Strontium Ranelate Reduces Fracture Risk
The TROPOS study of strontium ranelate (Protelos) reached the following conclusions about fracture risk reduction:
The number of patients experiencing a hip fracture was reduced by 36% (P=0.046) over 3 years of treatment in postmenopausal women over 74 years of age.
Protelos also reduced the relative risk of nonvertebral fracture by 16% (P=0.04) over 3 years compared with placebo.
Protelos reduced the risk of major fragility fractures (fracture of the hip, wrist, pelvis and sacrum, ribs and sternum, clavicle, humerus) by 19% (P=0.031) over 3 years compared with placebo.
In patients without prevalent vertebral fracture at baseline, Protelos reduced their risk of experiencing a first fracture by 45% (P<0.001).
The number of patients experiencing a hip fracture was reduced by 36% (P=0.046) over 3 years of treatment in postmenopausal women over 74 years of age.
Protelos also reduced the relative risk of nonvertebral fracture by 16% (P=0.04) over 3 years compared with placebo.
Protelos reduced the risk of major fragility fractures (fracture of the hip, wrist, pelvis and sacrum, ribs and sternum, clavicle, humerus) by 19% (P=0.031) over 3 years compared with placebo.
In patients without prevalent vertebral fracture at baseline, Protelos reduced their risk of experiencing a first fracture by 45% (P<0.001).
Thursday, November 5, 2009
BMD Improvements With Strontium
Research indicates that over three years, strontium can improve bone mineral density by 8-14%, depending on the site. These findings come from three studies on strontium ranelate (STRATOS, SOTI, and TROPOS). One study on strontium malonate (Strong study) showed a 2.66% increase in BMD at the lumbar spine after just three months. The University of California at Davis (UCD) is conducting a three-month clinical trial on strontium citrate, but the results may not be available before May, 2010.
The STRATOS trial (2002) of strontium ranelate determined that 680 mg strontium was the optimum dose. It was followed by a much larger study (SOTI study, 2004) of 1,649 osteoporotic postmenopausal women over a three-year period. Participants that received 680 mg of strontium daily, along with calcium and vitamin D supplements, increased lumbar bone mineral density by an average of 14.4% and femoral neck BMD an average of 8.3%.
The TROPOS study in 2005 focused on non-vertebral fractures in 5,091 postmenopausal women with osteoporosis. After five years, this double-blind, placebo-controlled study found an 8.2% improvement in the femoral neck and a 9.8% improvement in the total hip bone density.
In 2007, Osteologix, Inc. announced the results of its phase II clinical trial (Strong study) involving 289 postmenopausal women with low bone mineral density. The company reported that after three months, a 680 mg dose of strontium malonate increased lumbar spine BMD by 2.66%.
The STRATOS trial (2002) of strontium ranelate determined that 680 mg strontium was the optimum dose. It was followed by a much larger study (SOTI study, 2004) of 1,649 osteoporotic postmenopausal women over a three-year period. Participants that received 680 mg of strontium daily, along with calcium and vitamin D supplements, increased lumbar bone mineral density by an average of 14.4% and femoral neck BMD an average of 8.3%.
The TROPOS study in 2005 focused on non-vertebral fractures in 5,091 postmenopausal women with osteoporosis. After five years, this double-blind, placebo-controlled study found an 8.2% improvement in the femoral neck and a 9.8% improvement in the total hip bone density.
In 2007, Osteologix, Inc. announced the results of its phase II clinical trial (Strong study) involving 289 postmenopausal women with low bone mineral density. The company reported that after three months, a 680 mg dose of strontium malonate increased lumbar spine BMD by 2.66%.
Wednesday, September 23, 2009
Strontium Ranelate Safety
_ 3,790 patients were exposed to strontium ranelate during phase II and III trials. The overall incidence rates of adverse effects did not differ significantly from placebo. Adverse effects seen were generally mild and transient. The most common were:
_ headache (3.0% v 2.4%), nausea (6.6% v 4.3%), diarrhea (6.5% v 4.6%), loose stools (1.1% v 0.2%) dermatitis (2.1% v 1.6%) and eczema (1.5% v 1.2%)
_ In phase III studies, the annual incidence of venous thromboembolism (VTE) observed over 4 years was approximately 0.7%, with a relative risk of 1.42 (CI 1.02; 1.98, p=0.036) in strontium ranelate treated patients as compared to placebo treated patients. The cause of this finding is unknown. Strontium ranelate should be used with caution in patients at increased risk of VTE, including patients with a past history of VTE. The risk for strontium ranelate appears to be less than that seen with Selective Estrogen Receptor Modulator (SERM) or hormone replacement therapy (HRT).
_ Disturbances in consciousness, memory loss and seizures were all reported with higher frequency in the strontium ranelate group.
http://www.haad.ae/HAADDeps/Portals/7/Drug%20Monograph/strontium.ran%20final.pdf
_ headache (3.0% v 2.4%), nausea (6.6% v 4.3%), diarrhea (6.5% v 4.6%), loose stools (1.1% v 0.2%) dermatitis (2.1% v 1.6%) and eczema (1.5% v 1.2%)
_ In phase III studies, the annual incidence of venous thromboembolism (VTE) observed over 4 years was approximately 0.7%, with a relative risk of 1.42 (CI 1.02; 1.98, p=0.036) in strontium ranelate treated patients as compared to placebo treated patients. The cause of this finding is unknown. Strontium ranelate should be used with caution in patients at increased risk of VTE, including patients with a past history of VTE. The risk for strontium ranelate appears to be less than that seen with Selective Estrogen Receptor Modulator (SERM) or hormone replacement therapy (HRT).
_ Disturbances in consciousness, memory loss and seizures were all reported with higher frequency in the strontium ranelate group.
http://www.haad.ae/HAADDeps/Portals/7/Drug%20Monograph/strontium.ran%20final.pdf
Improvement Of Bone Microarchitecture By Strontium Ranelate
The analysis of transiliac bone biopsy samples from phase 2 and 3 clinical trials of strontium ranelate has provided further evidence of the good bone safety of strontium ranelate in the treatment of postmenopausal osteoporosis. Strontium ranelate improves both trabecular and cortical bone.
At the trabecular level, strontium ranelate significantly increases trabecular number by 14% and decreases trabecular separation by 16%, shifting trabeculae from rod-like structures to plate-like patterns. At the cortical level, strontium ranelate enlarges cortical bone dimensions by increasing cortical thickness by 18%.
Strontium ranelate is the first oral treatment to improve both trabecular and cortical bone in postmenopausal osteoporotic women. The change in 3D trabecular and cortical microarchitecture may improve bone biomechanical competence and explain the decreased fracture rate after strontium use.
http://www.servier.com/pro/osteoporosis/Osteoscoop/pdf/Osteoscoop_Issue61.pdf
At the trabecular level, strontium ranelate significantly increases trabecular number by 14% and decreases trabecular separation by 16%, shifting trabeculae from rod-like structures to plate-like patterns. At the cortical level, strontium ranelate enlarges cortical bone dimensions by increasing cortical thickness by 18%.
Strontium ranelate is the first oral treatment to improve both trabecular and cortical bone in postmenopausal osteoporotic women. The change in 3D trabecular and cortical microarchitecture may improve bone biomechanical competence and explain the decreased fracture rate after strontium use.
http://www.servier.com/pro/osteoporosis/Osteoscoop/pdf/Osteoscoop_Issue61.pdf
Monday, September 14, 2009
Measuring Risk Of Fracture
An individual's risk of fracture over a given period of years can be predicted using one of two models. Both are simple to use by individuals with no medical training. A patient simply answers a few questions.
The FRAX tool was developed by the World Health Organization (WHO) and gives the 10-year probability of hip fracture and the 10-year probability of a major osteoporotic fracture (spine, forearm, hip or shoulder fracture). It includes height, weight, personal history of fracture, family history of fracture, smoking, alcohol consumption, use of corticosteroids, rheumatoid arthritis and secondary osteoporosis. This model ignores falls. It is the most commonly used fracture-risk algorithm worldwide. To access it, follow this link, click on "Calculation Tool," and select your location and race/ethnicity:
http://www.shef.ac.uk/FRAX/
A second model is used in Australia to determine whether Pharmaceutical Benefits Scheme reimbursements for osteoporosis therapy apply. It was developed by the Garvan Institute of Medical Research in Sydney. The Garvan fracture risk calculator is based on gender, bone mineral density, age, history of personal fracture, and history of falls over the last 12 months. It is incredibly simple but is believed to incorporate the most critical risk factors. It provides five and 10 year risk assessments for hip fracture and for any osteoporosis/fragility fracture. The T-score and BMD in g/cm2 used in this tool refer to the values at the femoral neck, which will read Hip (neck) on most DXA scan reports. To access this calculator:
http://www.garvan.org.au/promotions/bone-fracture-risk/
The FRAX tool was developed by the World Health Organization (WHO) and gives the 10-year probability of hip fracture and the 10-year probability of a major osteoporotic fracture (spine, forearm, hip or shoulder fracture). It includes height, weight, personal history of fracture, family history of fracture, smoking, alcohol consumption, use of corticosteroids, rheumatoid arthritis and secondary osteoporosis. This model ignores falls. It is the most commonly used fracture-risk algorithm worldwide. To access it, follow this link, click on "Calculation Tool," and select your location and race/ethnicity:
http://www.shef.ac.uk/FRAX/
A second model is used in Australia to determine whether Pharmaceutical Benefits Scheme reimbursements for osteoporosis therapy apply. It was developed by the Garvan Institute of Medical Research in Sydney. The Garvan fracture risk calculator is based on gender, bone mineral density, age, history of personal fracture, and history of falls over the last 12 months. It is incredibly simple but is believed to incorporate the most critical risk factors. It provides five and 10 year risk assessments for hip fracture and for any osteoporosis/fragility fracture. The T-score and BMD in g/cm2 used in this tool refer to the values at the femoral neck, which will read Hip (neck) on most DXA scan reports. To access this calculator:
http://www.garvan.org.au/promotions/bone-fracture-risk/
Wednesday, August 19, 2009
Comparison of Strontium Malonate to Strontium Ranelate
Currently, Osteologix’s primary goal is to obtain approval for NB S101 (strontium malonate) for the treatment and prevention of osteoporosis. Their phase I study of the pharmacokinetic, or PK, properties of NB S101 revealed that a one gram tablet dose of NB S101 resulted in approximately the same level of strontium in human serum as a European company's approved product containing two grams of strontium ranelate in sachet formulation, which must be mixed with water before ingestion. Thus, at a significantly lower dose, their tablet formulation of strontium has shown bioequivalent levels of strontium to a marketed sachet product that has been proven safe and effective in osteoporotic patients in Europe.
More importantly, the recent results of their phase II study demonstrated that NB S101 decreased an established biomarker of bone resorption, CTX-1, in a dose-dependent manner by an amount statistically equivalent to or superior to the product approved in Europe. The phase II results also showed that NB S101 significantly increased bone mineral density at the lumbar spine and hip with only 12 weeks of treatment, and no significant side effects were noted in the trial.
More importantly, the recent results of their phase II study demonstrated that NB S101 decreased an established biomarker of bone resorption, CTX-1, in a dose-dependent manner by an amount statistically equivalent to or superior to the product approved in Europe. The phase II results also showed that NB S101 significantly increased bone mineral density at the lumbar spine and hip with only 12 weeks of treatment, and no significant side effects were noted in the trial.
Saturday, July 11, 2009
Improved T-Scores After Treatment
My first DEXA scan on 05/08/07 diagnosed me with osteoporosis. My second scan was done 07/06/09 after the following treatment: Fosamax 70 mg weekly from 06/18/07 to 12/24/07, two capsules Doctor's Best Strontium Bone Maker daily from 01/21/08 to 07/06/09. Here are my BMD results in g/cm2 and my T-scores for my second scan:
Spine (L1-L4): BMD 0.749, T-Score -2.7
Lt. Hip (neck): BMD 0.563, T-Score -2.6
Lt. Hip (total): BMD 0.739, T-Score -1.7
Despite my best efforts, my scores were not corrected for strontium intake by the radiologist. My first and second scans were not on the same DEXA machine, but they were both on DXA Hologic scanners. Even so, I am very happy with my results. My T-scores improved 10.0% at the spine, 7.1% at the left hip (neck), and 22.7% at the left hip (total). I plan to continue taking strontium citrate.
Spine (L1-L4): BMD 0.749, T-Score -2.7
Lt. Hip (neck): BMD 0.563, T-Score -2.6
Lt. Hip (total): BMD 0.739, T-Score -1.7
Despite my best efforts, my scores were not corrected for strontium intake by the radiologist. My first and second scans were not on the same DEXA machine, but they were both on DXA Hologic scanners. Even so, I am very happy with my results. My T-scores improved 10.0% at the spine, 7.1% at the left hip (neck), and 22.7% at the left hip (total). I plan to continue taking strontium citrate.
Monday, June 22, 2009
Study Shows Protelos Builds Better Bone Than Fosamax
A two-year double-blind study included 88 women over age 50 with postmenopausal osteoporosis who were treated with either Protelos (strontium ranelate) 2 g. daily or Fosamax (alendronate) 70 mg. weekly. The study, which used high-resolution computerized tomography, showed that Protelos increased cortical bone thickness, bone volume and trabecular bone density to a significantly greater extent than Fosamax over a one-year period.
The one-year interim results on bone microstucture, a determinant of bone strength, showed a +5.3% increase in cortical thickness and a +2.0% increase in bone volume in the Protelos-treated group. There was no change in the Fosamax-treated group.
For more information and references, see www.medicalnewstoday.com/printerfriendlynews.php?newsid=132149
The one-year interim results on bone microstucture, a determinant of bone strength, showed a +5.3% increase in cortical thickness and a +2.0% increase in bone volume in the Protelos-treated group. There was no change in the Fosamax-treated group.
For more information and references, see www.medicalnewstoday.com/printerfriendlynews.php?newsid=132149
Thursday, May 28, 2009
Update On Strontium Malonate For Osteoporosis
Osteologix, Inc. has received a key U.S. Patent Allowance for its osteoporosis drug, NB S101 (strontium malonate). This brings the company closer to its goal of manufacturing and marketing a prescription strontium drug in the U.S.
The Notice of Allowance from the U.S. Patent and Trademark Office is for U.S. Patent Application Number 11/269,289 titled "Water-Soluble Strontium Salts for Use in Treatment of Cartilage and/or Bone Conditions." It allows claims covering the treatment of osteoporosis and related bone conditions using NB S101 (strontium malonate). The patent will most likely issue in the second half of 2009 and expire in 2024.
The company has a number of other pending patent applications covering various aspects of the NB S101 drug program, including composition, manufacturing and method of use patent applications. "Based on the Notice of Allowance and the claims allowed by the USPTO, we believe the intellectual property protections established by this US patent allowance, coupled with our recently upheld European equivalent, will significantly enhance our ability to finalize strong development collaborations with potential partners to complete the Phase III development of NB S101 in osteoporosis," stated Philip J. Young, President and Chief Executive Officer of Osteologix. This is from the company's website at http://www.osteologix.com/.
The Notice of Allowance from the U.S. Patent and Trademark Office is for U.S. Patent Application Number 11/269,289 titled "Water-Soluble Strontium Salts for Use in Treatment of Cartilage and/or Bone Conditions." It allows claims covering the treatment of osteoporosis and related bone conditions using NB S101 (strontium malonate). The patent will most likely issue in the second half of 2009 and expire in 2024.
The company has a number of other pending patent applications covering various aspects of the NB S101 drug program, including composition, manufacturing and method of use patent applications. "Based on the Notice of Allowance and the claims allowed by the USPTO, we believe the intellectual property protections established by this US patent allowance, coupled with our recently upheld European equivalent, will significantly enhance our ability to finalize strong development collaborations with potential partners to complete the Phase III development of NB S101 in osteoporosis," stated Philip J. Young, President and Chief Executive Officer of Osteologix. This is from the company's website at http://www.osteologix.com/.
Update on Strontium Citrate Clinical Trial
BoneLady's Question, May 18, 2009:
What is the latest news on the strontium citrate for osteoporosis clinical trial? I went to the UCD site and found no update since February. I also visited www.clinicaltrials.gov/ and did not find this study listed. Is the medical center still recruiting? If so, what is the projected start date for the trial?
SCOPE Study Coordinator's Reply, May 27, 2009:
At this time, we are recruiting and enrolling participants and will most likely be doing so through the end of the year. No results will be available until at least this time next year.
What is the latest news on the strontium citrate for osteoporosis clinical trial? I went to the UCD site and found no update since February. I also visited www.clinicaltrials.gov/ and did not find this study listed. Is the medical center still recruiting? If so, what is the projected start date for the trial?
SCOPE Study Coordinator's Reply, May 27, 2009:
At this time, we are recruiting and enrolling participants and will most likely be doing so through the end of the year. No results will be available until at least this time next year.
Monday, May 11, 2009
Strontium and Antibiotics
Strontium may reduce the absorption of tetracycline antibiotics (e.g., tetracycline, minocycline, doxycycline) and quinolone antibiotics (e.g., ciprofloxacin, norfloxacin, nalidixic acid) from the gut and could make them less effective.
If you are prescribed a course of one of these types of antibiotics you should stop taking strontium during the course of antibiotics.This information is from www.netdoctor.co.uk/medicines/100000762.html
If you are prescribed a course of one of these types of antibiotics you should stop taking strontium during the course of antibiotics.This information is from www.netdoctor.co.uk/medicines/100000762.html
Labels:
antibiotics,
osteoporosis,
quinolone,
strontium,
tetracycline
Monday, May 4, 2009
Poll Results On T-Scores After Strontium Citrate
Here are the results of my latest poll: Did your DEXA scan T-scores improve after taking strontium citrate?
12 (75%) YES
4 (25%) NO
12 (75%) YES
4 (25%) NO
Labels:
DEXA scan,
Strontium citrate,
T-scores
Friday, April 17, 2009
Strontium Safety
Strontium is safe for most people, but there are certain individuals who should not take it. These include those with any of the following disorders:
- Decreased kidney function.
- History of blood clots in the veins (venous thromboembolism, e.g., deep vein thrombosis or pulmonary embolism).
- Blood disorders that increase the risk of blood clots in the veins, e.g. antiphospholipid syndrome, factor V Leiden.
There is some evidence that strontium could slightly increase the incidence of blood clots. The tests were performed on strontium ranelate.
Strontium should also not be taken by children nor by pregnant or lactating women.
- Decreased kidney function.
- History of blood clots in the veins (venous thromboembolism, e.g., deep vein thrombosis or pulmonary embolism).
- Blood disorders that increase the risk of blood clots in the veins, e.g. antiphospholipid syndrome, factor V Leiden.
There is some evidence that strontium could slightly increase the incidence of blood clots. The tests were performed on strontium ranelate.
Strontium should also not be taken by children nor by pregnant or lactating women.
Wednesday, April 8, 2009
A Dose-Response Study With Strontium Malonate
Osteologix, Inc. is conducting a Phase II clinical trial with strontium malonate in postmenopausal women. The study's primary objective is to compare dose-response effect of three levels of strontium malonate to placebo on bone resorption quantified by S-CTX-1 following 12 weeks of treatment. The four parallel groups in this trial will take 750 mg strontium malonate, 1000 mg strontium malonate, 2000 mg strontium malonate, 2 grams Protelos (strontium ranelate), and placebo. For more details, visit http://clinicaltrials.gov/ct2/show/NCT00409032
Tuesday, April 7, 2009
FDA Bisphosphonate Alert
Information on Bisphosphonates (marketed as Actonel, Actonel+Ca, Aredia, Boniva, Didronel, Fosamax, Fosamax+D, Reclast, Skelid, and Zometa)
FDA ALERT [1/7/2008] - FDA is highlighting the possibility of severe and sometimes incapacitating bone, joint, and/or muscle (musculoskeletal) pain in patients taking bisphosphonates. Although severe musculoskeletal pain is included in the prescribing information for all bisphosphonates, the association between bisphosphonates and severe musculoskeletal pain may be overlooked by healthcare professionals, delaying diagnosis, prolonging pain and/or impairment, and necessitating the use of analgesics.
The severe musculoskeletal pain may occur within days, months, or years after starting a bisphosphonate. Some patients have reported complete relief of symptoms after discontinuing the bisphosphonate, whereas others have reported slow or incomplete resolution. The risk factors for and incidence of severe musculoskeletal pain associated with bisphosphonates are unknown.
This severe musculoskeletal pain is in contrast to the acute phase response characterized by fever, chills, bone pain, myalgias, and arthralgias that sometimes accompanies initial administration of intravenous bisphosphonates and may occur with initial exposure to once-weekly or once-monthly doses of oral bisphosphonates. The symptoms related to the acute phase response tend to resolve within several days with continued drug use.
Healthcare professionals should consider whether bisphosphonate use might be responsible for severe musculoskeletal pain in patients who present with these symptoms and consider temporary or permanent discontinuation of the drug.
This information reflects FDA's current analysis of data available to FDA concerning this drug. FDA intends to update this when additional information or analyses become available.
FDA ALERT [1/7/2008] - FDA is highlighting the possibility of severe and sometimes incapacitating bone, joint, and/or muscle (musculoskeletal) pain in patients taking bisphosphonates. Although severe musculoskeletal pain is included in the prescribing information for all bisphosphonates, the association between bisphosphonates and severe musculoskeletal pain may be overlooked by healthcare professionals, delaying diagnosis, prolonging pain and/or impairment, and necessitating the use of analgesics.
The severe musculoskeletal pain may occur within days, months, or years after starting a bisphosphonate. Some patients have reported complete relief of symptoms after discontinuing the bisphosphonate, whereas others have reported slow or incomplete resolution. The risk factors for and incidence of severe musculoskeletal pain associated with bisphosphonates are unknown.
This severe musculoskeletal pain is in contrast to the acute phase response characterized by fever, chills, bone pain, myalgias, and arthralgias that sometimes accompanies initial administration of intravenous bisphosphonates and may occur with initial exposure to once-weekly or once-monthly doses of oral bisphosphonates. The symptoms related to the acute phase response tend to resolve within several days with continued drug use.
Healthcare professionals should consider whether bisphosphonate use might be responsible for severe musculoskeletal pain in patients who present with these symptoms and consider temporary or permanent discontinuation of the drug.
This information reflects FDA's current analysis of data available to FDA concerning this drug. FDA intends to update this when additional information or analyses become available.
Labels:
Actonel,
alert,
Aredia,
bisphosphonates,
bone pain,
Boniva,
Didronel,
Fosamax,
joint pain,
muscle pain,
Reclast,
Skelid,
Zometa
Tuesday, March 31, 2009
Dieters Risk Bone Loss
"What happens to bones during weight loss?" " It depends on how you lose the weight. A study conducted at the Washington University School of Medicine in St. Louis monitored two groups of people who lost a little more than one pound per month for a year. Half of them lost the weight by eating fewer calories, and the other half lost by increasing their physical activity. Despite the gradual rate of weight loss, the dieters lost bone mineral density from their spines and hips. The exercisers lost weight but not bone mass." For more on this subject, see http://archives.starbulletin.com/2008/03/08/features/health.html.
Labels:
bone mass,
bone mineral density,
diet,
dieters,
exercise,
exercisers,
weight loss
Not All Strontium Supplements Are The Same
Be careful about the strontium supplement you choose. They are not all the same. The optimum dose is 680 mg elemental strontium daily. One study found that three out of five products tested contained significantly less strontium than their labels indicated. The two properly labeled products were AOR Strontium Support and Strontium Bone Maker. The information from the study came from
http://www.starbulletin.com/specialprojects/09/yah/20090326_supplement_helps_bones_grow_stronger.html
http://www.starbulletin.com/specialprojects/09/yah/20090326_supplement_helps_bones_grow_stronger.html
Labels:
bones,
strontium,
supplements
Friday, March 6, 2009
Blood and Urine Calcium
Strontium interferes with colorimetric methods for the determination of blood and urinary calcium concentrations. Therefore, in medical practice, inductively coupled plasma atomic emission spectrometry or atomic absorption spectrometry methods should be used to ensure an accurate assessment of blood and urinary calcium concentrations. These guidelines are included in the electronic Medicines Compendium (eMC), which contains information about UK licensed medicines:
http://emc.medicines.org.uk/document.aspx?documentID=15410
http://emc.medicines.org.uk/document.aspx?documentID=15410
Adjustments To BMD Unnecessary With Strontium Therapy
Per Servier, the manufacturer of Protelos (strontium ranelate), their product increases BMD and decreases the risk of vertebral fracture. They also state on their website at www.servier.com, that doctors do not need to adjust BMD measurements in individual patients:
How much does Protelos increase BMD?
"The increase in BMD with Protelos is superior to that of other treatments. It is proven that Protelos is effective against vertebral and hip fractures, and a correlation up to 74% has been established between the increase in BMD with Protelos and vertebral fracture risk reduction, therefore it is not necessary to adjust BMD for each patient. This serves as a tool to measure compliance (allowing you to confirm that your patient is taking treatment) and a marker of clinical efficacy (for motivating them to continue taking treatment)."
In practice do I have to adjust BMD measurements in individual patients?
"No, it is not necessary to adjust BMD for each patient because each increase in BMD is highly correlated (up to 74%) with the decrease in the risk of sustaining a vertebral fracture. In other words with Protelos, the more the increase of BMD in your patient, the more your patient is protected from fracture. Moreover, BMD is a useful monitoring tool to confirm the compliance of your patient."
How much does Protelos increase BMD?
"The increase in BMD with Protelos is superior to that of other treatments. It is proven that Protelos is effective against vertebral and hip fractures, and a correlation up to 74% has been established between the increase in BMD with Protelos and vertebral fracture risk reduction, therefore it is not necessary to adjust BMD for each patient. This serves as a tool to measure compliance (allowing you to confirm that your patient is taking treatment) and a marker of clinical efficacy (for motivating them to continue taking treatment)."
In practice do I have to adjust BMD measurements in individual patients?
"No, it is not necessary to adjust BMD for each patient because each increase in BMD is highly correlated (up to 74%) with the decrease in the risk of sustaining a vertebral fracture. In other words with Protelos, the more the increase of BMD in your patient, the more your patient is protected from fracture. Moreover, BMD is a useful monitoring tool to confirm the compliance of your patient."
Labels:
BMD,
BMD adjustments,
Protelos,
strontium ranelate
Monday, March 2, 2009
Strontium Citrate Clinical Trial
UC Davis Study to Prevent Osteoporosis with Dietary Supplement Begins Recruitment
http://www.ucdmc.ucdavis.edu/newsroom/newsdetail.html?key=1985
February 26, 2009(SACRAMENTO, Calif.) — Osteoporosis affects many women and can cause painful, disabling and even life-threatening fractures. Researchers from the UC Davis Department of Internal Medicine are seeking a simple, inexpensive way to prevent the disease.
Strontium citrate is a widely available, over-the-counter dietary supplement promoted to “improve bone health.” Strontium is a natural element found in bone in all people. Strontium citrate is another form of strontium ranelate, a proven medication prescribed across Europe and Australia to treat and prevent osteoporosis and related fractures. Unlike pharmaceuticals, strontium citrate is not a prescribed medication and is inexpensive.
The UC Davis researchers are trying to demonstrate that a nutraceutical, which women can buy without seeing a doctor or paying a drug company, can be used to improve bone health.
The researchers are seeking post-menopausal women who are at least one year but less than five years past their last menstrual period. Participation in the study will include a screening visit with blood draw at UC Davis Medical Center, followed by a blood draw and free DEXA scan at the Veterans Administration Northern California Health Care Center.
Participants will be randomly assigned to one of two groups. One group will take strontium citrate plus calcium and vitamin D for three months, while the other group will take a placebo plus calcium and vitamin D for three months.
During the three-month period, participants will visit UC Davis Medical Center three times for short questionnaires and blood draws. DEXA scans and test results can be provided to participants.For more information or to schedule a screening visit, contact Stephanie Burns, study coordinator, at (530) 754-7576 or (916) 734-5562 or scope@phs.ucdavis.edu.
http://www.ucdmc.ucdavis.edu/newsroom/newsdetail.html?key=1985
February 26, 2009(SACRAMENTO, Calif.) — Osteoporosis affects many women and can cause painful, disabling and even life-threatening fractures. Researchers from the UC Davis Department of Internal Medicine are seeking a simple, inexpensive way to prevent the disease.
Strontium citrate is a widely available, over-the-counter dietary supplement promoted to “improve bone health.” Strontium is a natural element found in bone in all people. Strontium citrate is another form of strontium ranelate, a proven medication prescribed across Europe and Australia to treat and prevent osteoporosis and related fractures. Unlike pharmaceuticals, strontium citrate is not a prescribed medication and is inexpensive.
The UC Davis researchers are trying to demonstrate that a nutraceutical, which women can buy without seeing a doctor or paying a drug company, can be used to improve bone health.
The researchers are seeking post-menopausal women who are at least one year but less than five years past their last menstrual period. Participation in the study will include a screening visit with blood draw at UC Davis Medical Center, followed by a blood draw and free DEXA scan at the Veterans Administration Northern California Health Care Center.
Participants will be randomly assigned to one of two groups. One group will take strontium citrate plus calcium and vitamin D for three months, while the other group will take a placebo plus calcium and vitamin D for three months.
During the three-month period, participants will visit UC Davis Medical Center three times for short questionnaires and blood draws. DEXA scans and test results can be provided to participants.For more information or to schedule a screening visit, contact Stephanie Burns, study coordinator, at (530) 754-7576 or (916) 734-5562 or scope@phs.ucdavis.edu.
Labels:
clinical trial,
osteoporosis,
Strontium citrate
Friday, February 27, 2009
Strontium and Osteoporosis
On July 7, 2008, Sara S. DeHart, MSN, Ph.D., Online Journal Contributing Writer, wrote Strontium and Osteoporosis: A Treatment Not Offered to American Women. "The purpose of this article is to provide a summary of current published research on the mineral strontium and its purported function in preventing osteoporotic fractures in postmenopausal women. This mineral is available through regular medical sources in Europe and is approved for use in 21 European countries." For comparative purposes of what happens to postmenopausal women in the United States, she included a case study of her own "journey through this morass of data and treatment options." Because Dr. DeHart's stated purpose in writing the article so closely correlates with my purpose in maintaining this blog, I have added a link to her article. http://onlinejournal.com/artman/publish/article_3458.shtml
Labels:
American women,
osteoporosis treatment,
strontium
Friday, January 30, 2009
Poll Results
Here are the results of my previous poll, with 104 responses: Are you currently taking strontium for bone problems?
Yes 80 (76%)
No 24 (23%)
Please take part in the current poll on DEXA scan results after strontium therapy.
Yes 80 (76%)
No 24 (23%)
Please take part in the current poll on DEXA scan results after strontium therapy.
Labels:
bone problems,
poll,
strontium
Subscribe to:
Posts (Atom)
Wandering Skeleton

Artist: Joel Hoekstra
Osteoporotic Bone
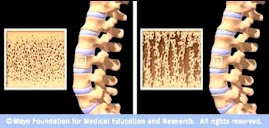
Source: www.mayoclinic.com
How Strontium Builds Bones
Strontium is a mineral that tends to accumulate in bone. Studies have shown that oral doses of strontium are a safe and effective way to prevent and reverse osteoporosis. Doses of 680 mg per day appear to be optimal. See my "For More Information About Strontium" links section.
Osteoporosis is caused by changes in bone production. In healthy young bones there is a constant cycle of new bone growth and bone removal. With age, more bone is removed and less new bone is produced. The bones become less dense and thus more fragile.
Scientists believe that strontium works in two ways. It may stimulate the replication of pre-osteoblasts, leading to an increase in osteoblasts (cells that build bone). Strontium also directly inhibits the activity of osteoclasts (cells that break down bone). The result is stronger bones.
When taking strontium, be sure to take 1200 mg calcium, 1000 IU vitamin D3, and 500 mg magnesium daily. It is best to take strontium late at night on an empty stomach. Calcium and strontium may compete with each other for absorption if taken together.
Osteoporosis is caused by changes in bone production. In healthy young bones there is a constant cycle of new bone growth and bone removal. With age, more bone is removed and less new bone is produced. The bones become less dense and thus more fragile.
Scientists believe that strontium works in two ways. It may stimulate the replication of pre-osteoblasts, leading to an increase in osteoblasts (cells that build bone). Strontium also directly inhibits the activity of osteoclasts (cells that break down bone). The result is stronger bones.
When taking strontium, be sure to take 1200 mg calcium, 1000 IU vitamin D3, and 500 mg magnesium daily. It is best to take strontium late at night on an empty stomach. Calcium and strontium may compete with each other for absorption if taken together.
For More Information about Strontium
- A Dose-response Study With Strontium Malonate
- A Review of the latest insights into the mechanism of action of strontium in bone
- Antifracture Efficacy Over 10 Years With Strontium Ranelate
- Combination of Micronutrients for Bone (COMB) Study: Bone Density after Micronutrient Intervention
- Effect of bone strontium on BMD measurements
- Effects of SrR on Calcium Metabolism
- Effects of strontium ions on growth and dissolution of hydroxyapatite and on bone mineral detection
- Influence of strontium on bone mineral density and bone mineral content measurements by dual X-ray absorptiometry
- Interpretation of BMD Scans in Patients Stopping Strontium
- Melatonin-micronutrients Osteopenia Treatment Study (MOTS)
- National Osteoporosis Foundation
- Osteoporosis And Bone Physiology
- P471 BENEFICIAL EFFECTS OF STRONTIUM RANELATE COMPARED TO ALENDRONATE ON TRABECULAR BONE SCORE IN POST MENOPAUSAL OSTEOPOROTIC WOMEN: A 2-YEAR STUDY
- Post-Marketing Assessment of the Safety of Strontium Ranelate
- PubMed Abstract On The SOTI Study
- PubMed Abstract On The TROPOS Study
- Strontium ranelate Aristo
- Strontium Ranelate For Spinal Osteoarthritis
- Strontium: Breakthrough Against Osteoporosis
- Summary Safety Review - Strontium
- Thirteen Key Diagnostic Tests